Individual Medication Preparation (IMP), and more specifically Automated Dose Dispensing (ADD) needs to be notified to the Federal Agency for Medicines and Health Products (FAMHP). A list of approved pharmacies with ADD is available on the site of FAMHP.
A Royal Decree (RD of September 24th 2012) establishing the regulations IMP describes the conditions and requirements that a pharmacy wishing to perform IMP must meet.
IMP involves preparing a package containing one/more products intended for individual administration to a specific patient at a specific time. The products may be drugs and/or food supplements. IMP can be automated (ADD) or manual.
The marketing authorization holder remains responsible for the quality of the product in the closed original container (during the shelf life and as long as the storage conditions are respected).
The registered pharmacist (authorization holder) who performs the IMP is responsible for all changes that are made during the IMP process with respect to the authorized drug and which may affect the quality of the drug (storage outside the original packaging, combining with other drugs or food supplements, labeling IMP packaging, etc.).
Medication and/or food supplements needed for IMP are purchased in the necessary quantities. Depending on the scale of the operations, a stock can be built, however this should be done rationally (no unnecessary stock), and expiry dates should be taken into account. Suppliers should be approved.

In the best case the products purchased are bulk products, however, in most cases the products are delivered in the classic secondary packaging selling unit. This means that in order to dispense them by patient, blistered products must be deblistered. There must be attention to line clearance beforehand so as not to obtain a mix up. Never should different lot numbers be mixed (traceability is required!)Bulk products are kept in a sealed container, containing: product name, strength, lot number and status (quarantined, released).The obtained bulk products are controlled and released by the registered pharmacist.N.B The shelf life of bulk products is difficult to determine: at best, the manufacturer provides recommendations on bulk shelf life. In other cases, shelf life can be determined on physical-chemical properties (see e.g. SmPC, Ph. Eur.), with a maximum of 12 months (and not exceeding the shelf life of the original packaging)When multiple containers are available for the same product, the FEFO (First expired, first out) principle is used. These containers are used to fill the canisters of the ADD equipment.

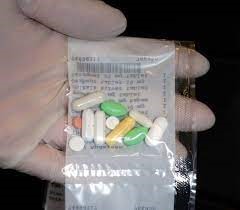
Before any dispensing operation is started, a line clearance should be performed. ADD equipment contains multiple canisters. Each canister only contains one medicinal product name and strength and is calibrated for this product. Based on the administration schedule of the patients, the ADD equipment picks the relevant canisters and prepares the final dispensed dose. Each dose packaged must be free of air and transparent.
In case of patient-specific manual addition, a double check is always required by a second operator.

A combination of automated and visual checking of ADD packaging is recommended. The number and identity, the integrity of the container and the labelling should be checked.
Typical errors are: tablet too much or too little in the dose, contamination / broken tablet, tablet is not recognized…
A correction can be made to the errors discovered during checking. ADD packagings which have been opened as part of this correction, shall be marked as such and carefully resealed.
At the end of each ADD production run, a check is made on the correct execution of the process. This check is performed by, or under the responsibility of, the registered pharmacist.
The release of the ADD packages is done by the registered pharmacist and confirmed by a signature on the protocol.
There’re of course specificities, requirements and precautionary measures that need to be taken into account by the registered pharmacist before dispensing IMP. Q-support is happy to assist you in setting up and/or managing an appropriate IMP Quality Management System.
Do you want to receive our interesting blogs directly into your email? Subscribe to our newsletter!